Dr. Gayden's Chemistry Class
Day-to-day activities from Dr. Gayden's Chemistry class at Dorothy M. Wallace COPE Center.
Monday, February 4, 2019
Monday, January 14, 2019
Tuesday, 15 January, 2019
Ready to "Escape" learning about how to name ionic and covalent compounds? Then, escape this room and you'll be an expert!
Mission Escape!
Here is the long link so that you can get the code anywhere!
Access This If You Want To Learn How To Name Compounds!
Mission Escape!
Here is the long link so that you can get the code anywhere!
Access This If You Want To Learn How To Name Compounds!
Tuesday, December 19, 2017
Tuesday through Friday, 19 - 22 December, 2017
ESSENTIAL QUESTION: What keeps substances together?
LEARNING TARGET: What are attractive forces?
BENCHMARKS: SC.912.P.8.6
LEARNING OBJECTIVES: Students will be able to:
-Describe why atoms come together to form chemical bonds.
-Differentiate between hydrogen bonding and van der Walls forces.
BELL RINGER - You have two pieces of paper that you want to bind together. List all the possible ways you could do this.
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: notebook update
AGENDA
WHOLE GROUP
NOTE: Due to Holiday celebrations, some class activities may not occur.
For the bell ringer, students listed all the possible ways to bind two sheets of paper together.
We did a teacher demo for hydrogen bonds.
Students then saw the video Atomic Hook -Ups. You can watch the video by clicking the link below.
Students then did the online activity determining bond types. Visit my blog page for December 19/20 (the other one!) for the link!
For an early Christmas present, I'll also include it below: :-)
Monday, December 18, 2017
Thursday, December 14, 2017
Friday/Monday, 15/18 December, 2017
Click on the link below to access the activity.
It's Elementary: Building Elements
Atomic Electron Configuration
It's Elementary: Building Elements
Atomic Electron Configuration
Monday, December 11, 2017
Monday through Monday, 11 through 20 December, 2017
ESSENTIAL QUESTION: How are electrons arranged in atoms?
LEARNING TARGET: Identify elements by their electron configuration
BENCHMARKS: SC.912.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-explain that when electrons transition to higher energy levels they absorb energy, and emit energy when they transition to lower energy levels.
-Take a quiz on electron configuration
-Data chat
BELL RINGER - short study session
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: notebook update
AGENDA
WHOLE GROUP
Note: Due to Holiday activities and Field Trips, these assignments may extend into other class periods.
Students had a short study session before the exam on electron configuration.
Students then either took the exam on electron configuration and/or corrected exams already taken.
The remainder of class time was spent doing individual assignments, including completing the Electron Configuration Gizmo.
Thursday, December 7, 2017
Tuesday - Friday, 5-8 December, 2017
ESSENTIAL QUESTION: How are electrons arranged in atoms?
LEARNING TARGET: Identify the metallic elements by their characteristic colors in a flame test.
BENCHMARKS: SC.912.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-explain that when electrons transition to higher energy levels they absorb energy, and emit energy when they transition to lower energy levels.
-Data chat
BELL RINGER - identify the elements:
- 1s2 2s2 2p6 3s2 3p3 -
- 1s22s22p63s23p64s23d104p65s1.
or on Thursday/Friday: Write the short hand notation for the electron configuration of: copper, silver, and francium
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: notebook update
AGENDA
WHOLE GROUP
Students completed the above bell ringers on the appropriate days.
Students completed the lab write up during class. We also discussed the rules and exceptions of how to write electron configurations, including the short hand method.
Students also completed the Gizmo on electron configuration, along with the assessment that accompanies it.
Sunday, December 3, 2017
Friday/Monday, 01/04 December, 2017
ESSENTIAL QUESTION: How are electrons arranged in atoms:
LEARNING TARGET: Identify the metallic elements by their characteristic colors in a flame test.
BENCHMARKS: SC.912.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-explain that when electrons transition to higher energy levels they absorb energy, and emit energy when they transition to lower energy levels.
-Data chat
BELL RINGER - Read background info and develop a problem statement.
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: notebook update
AGENDA
WHOLE GROUP
Students were asked to read the introduction for the lab as their bell ringer, in order to develop a problem statement.
HL 4 was collected and reviewed.
Students set up their lab notebooks for the Flame Test lab. Students then completed the lab portion of the lab. We will write up the remainder of the lab next class.
Wednesday, November 29, 2017
Wednesday/Thursday, 29/30 November, 2017
ESSENTIAL QUESTION: How are electrons arranged in atoms:
LEARNING TARGET: Write the correct electron configuration for various elements on the periodic table.
BENCHMARKS: SC.912.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-apply basic rules of electron configuration to determine the valence electrons of an atom and their chemical and physical properties.
-explain that when electrons transition to higher energy levels they absorb energy, and emit energy when they transition to lower energy levels.
-Data chat
BELL RINGER - Write the electron configurations for Ne, Cl, and Na.
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: HL 4: Electron Configuration
AGENDA
WHOLE GROUP
Students wrote the electron configurations for Ne, Cl, and Na as their bell ringer.
Students then took notes on Electron Configuration. You can see a movie of the slide show below.
Students also received handouts that will become part of their notes, explaining the different rules for writing electron configurations. You can find these handouts below.
Monday, November 27, 2017
Monday/Tuesday, 27/28 November, 2017
ESSENTIAL QUESTION: How are electrons arranged in atoms:
LEARNING TARGET: Write the correct electron configuration for various elements on the periodic table.
BENCHMARKS: SC.912.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-apply basic rules of electron configuration to determine the valence electrons of an atom and their chemical and physical properties.
-explain that when electrons transition to higher energy levels they absorb energy, and emit energy when they transition to lower energy levels.
-Data chat
BELL RINGER - Write the electron configurations for H, He and Li.
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: notebook kupdate
AGENDA
WHOLE GROUP
Students wrote the electron configurations for H, He, and Li as their bell ringer.
Students who needed to make up QSBA1 did so, while others worked independently or had data chats.
Students then turned in HL 3 and we reviewed it.
Students then worked on their Gizmos.
Monday, November 20, 2017
Monday/Tuesday, 20/21 November, 2017
ESSENTIAL QUESTION: How are electrons arranged in atoms:
LEARNING TARGET: Write the correct electron configuration for various elements on the periodic table.
BENCHMARKS: SC.912.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-apply basic rules of electron configuration to determine the valence electrons of an atom and their chemical and physical properties.
-explain that when electrons transition to higher energy levels they absorb energy, and emit energy when they transition to lower energy levels.
-QSBA1 make up/debrief/correction
BELL RINGER - NA.
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: HL4: Electron Configuration
AGENDA
WHOLE GROUP
I was out last class, and period 6 did not get HL , which they received today. They also received the I Heart Electron Configuration words and viewed the video. These things can be seen in the last post.
Both classes were cut short due to the activities of Spirit Week.
Thursday, November 16, 2017
Thursday/Friday, 16/17 November, 2017
ESSENTIAL QUESTION: How are electrons arranged in atoms:
LEARNING TARGET: Write the correct electron configuration for various elements on the periodic table.
BENCHMARKS: SC.912.P.8.5
LEARNING OBJECTIVES: Students will be able to:
-apply basic rules of electron configuration to determine the valence electrons of an atom and their chemical and physical properties.
-explain that when electrons transition to higher energy levels they absorb energy, and emit energy when they transition to lower energy levels.
-QSBA1 make up/debrief/correction
BELL RINGER - NA.
VOCABULARY: energy, ground state, excited state, emission spectra, photon, Planck’s constant, quantum, quantum mechanical model, electron configuration, atomic orbital, energy levels, Aufbau Principle, Pauli Exclusion Principle, Hund’ Rule
HOME LEARNING: HL4: Electron Configuration
AGENDA
WHOLE GROUP
We did not have a bell ringer. Students received home learning 3 and we reviewed the principles behind it.
Students who needed to make up QSBA1 did so. Other students corrected their exams.
Students then watched the video I Heart Electron Configuration and sang along with the words below. You can find the video and the words below.
Thursday, November 9, 2017
Thursday, 09 November through Wednesday, 15 November, 2017
Note: Students will take the QSBA 1 exam on:
Period 2: November 13
Period 3: November 14
Period 2: November 13
Period 3: November 14
ESSENTIAL QUESTION: What is the electromagnetic spectrum?
LEARNING TARGET: How do I interact with the electromagnetic spectrum?
BENCHMARKS: SC.912.P.10.1
LEARNING OBJECTIVES: Students will be able to:
-Identify the general properties of all electromagnetic waves.
-compare and contrast the types of radiation in the electromagnetic spectrum according to wavelength and frequency.
-Student data chat.
BELL RINGER - on test day - 15 min study session
on non test day - Read the Wavestown Descriptions
VOCABULARY: wavelength, frequency, speed, electromagnetic spectrum, hertz
HOME LEARNING: notebook update/study for assessment
AGENDA
WHOLE GROUP
On Thursday, students read the Wavestown description and completed the electromagnetic spectrum handout. They then labeled the Wavestron diagram.
Students needed more practice solving equation. We did several whole class examples.
On Monday, Period 6 took the QSBA1 exam. Period 7 took the exam on Tuesday. Make-up exams will be given on Wednesday and Thursday.
While others are testing, students that have completed testing should work on their Gizmo (Electron Configuration) while we do data chats.
On Monday, Period 6 took the QSBA1 exam. Period 7 took the exam on Tuesday. Make-up exams will be given on Wednesday and Thursday.
While others are testing, students that have completed testing should work on their Gizmo (Electron Configuration) while we do data chats.
Tuesday, November 7, 2017
Tuesday/Wednesday, 07/08 November, 2017
ESSENTIAL QUESTION: What is the electromagnetic spectrum?
LEARNING TARGET: How do I interact with the electromagnetic spectrum?
BENCHMARKS: SC.912.P.10.1
LEARNING OBJECTIVES: Students will be able to:
-Identify the general properties of all electromagnetic waves.
-compare and contrast the types of radiation in the electromagnetic spectrum according to wavelength and frequency.
-Student data chat.
BELL RINGER - Read the Wavestown Descriptions
VOCABULARY: wavelength, frequency, speed, electromagnetic spectrum, hertz
HOME LEARNING: HL 2: The Electromagnetic Spectrum
AGENDA
WHOLE GROUP
Students read the Wavestown description as their bell ringer.
They then Watched a BrainPop on the Electromagnetic Spectrum. You can watch the video by click the link below and signing in with the user name amsbartow and the password ams13.
Students then practiced solving word problems for speed, frequency and wavelength.
Finally students completed the handouts for Wavestown. You can find them below.
Students also completed the questions for the Bohr Introduction Gizmo.
Friday, November 3, 2017
Friday/Monday, 03/06 November, 2017
ESSENTIAL QUESTION: How has the Atomic Model changed over time?
LEARNING TARGET: Describe how the modern atomic theory was developed.
BENCHMARKS: SC.912.P.8.3
LEARNING OBJECTIVES: Students will be able to:
-Describe the structure of atoms in terms of protons, neutrons, and electrons, and differentiate among these particles in terms of mass, electrical changes and location within the atom.
-Student data chat.
BELL RINGER - Complete the handout Your guide to the atom
VOCABULARY: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas, notation, nonmetal, octet rule
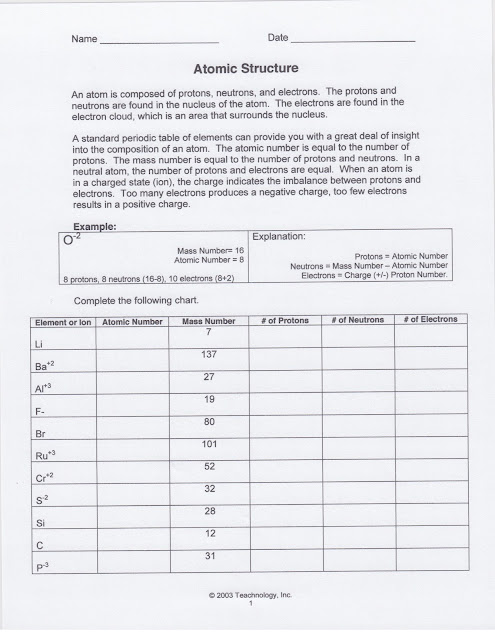
HOME LEARNING: HL 1: Atomic Structure
AGENDA
WHOLE GROUP
Students completed the handout Your Guide to the Atom.
Students received HL 1 Atomic Structure. It is due next class period. You can find the copy of the handout above.
We reviewed how to determine the number of protons, neutrons, and electrons in both charged and neutral atoms. We also determined how to calculate the number of neutrons using the atomic mass of the element.
Students then worked on the Bohr's Introduction model Gizmo. Be sure to complete the five question assessment at the end fo the GIZMO.
Wednesday, November 1, 2017
Wednesday/Thursday, 01/02 November, 2017
ESSENTIAL QUESTION: How has the Atomic Model changed over time?
LEARNING TARGET: Describe how the modern atomic theory was developed.
BENCHMARKS:SC.912.P.8.3
LEARNING OBJECTIVES: Students will be able to:
-Describe the structure of atoms in terms of protons, neutrons, and electrons, and differentiate among these particles in terms of mass, electrical changes and location within the atom.
-Student data chat.
BELL RINGER - Complete the handout Your guide to the atom
VOCABULARY: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas, notation, nonmetal, octet rule
HOME LEARNING: update notebook
AGENDA
WHOLE GROUP
Those students who needed to completed Your Guide to the Atom.
Students also worked on the construction of their project boards.
Other students worked on the Borh's Model Gizmo. Students are to take the assessment after completing the Gizmo.
Monday, October 30, 2017
Monday/Tuesday, 30/31 October, 2017
ESSENTIAL QUESTION: How has the Atomic Model changed over time?
LEARNING TARGET: Describe how the modern atomic theory was developed.
BENCHMARKS:: SC.912.P.8.3
LEARNING OBJECTIVES: Students will be able to:
-Describe the structure of atoms in terms of protons, neutrons, and electrons, and differentiate among these particles in terms of mass, electrical changes and location within the atom.
-Student data chat.
BELL RINGER - Complete the handout Your guide to the atom
VOCABULARY: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas, notation, nonmetal, octet rule
HOME LEARNING: update notebook
AGENDA
WHOLE GROUP
Students completed the handout Your Guide to the Atom as their bell ringer.
Students then retook a base-line exam for Chemistry, one that is from the District, for end of year comparisons.
Students then used class time to work on their element presentations.
Wednesday, October 25, 2017
Thursday/Friday, 25/26 October, 2017
NOTE _ Period 6 did not meet on Thursday due to the Fall Harvest Festival. They completed all work on Tuesday. See that blog to determine what you missed.
ESSENTIAL QUESTION: How has the Atomic Model changed over time?
LEARNING TARGET: Describe how the modern atomic theory was developed.
BENCHMARKS: SC.912.P.8.3
LEARNING OBJECTIVES: Students will be able to:
-Complete the Separation of Mixtures lab write up.
-Describe the atomic theory and the reasons for the changes in the model over time.
-Describe the structure of atoms in terms of protons, neutrons, and electrons, and differentiate among these particles in terms of mass, electrical changes and location within the atom.
BELL RINGER - Your guide to the atom
VOCABULARY: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas, notation, nonmetal, octet rule
HOME LEARNING: update notebook
AGENDA
WHOLE GROUP
Students labeled the atom as their bell ringer.
We then completed notes (see last blog for move of power point) for those students who needed to complete them.
Students also had the chance to complete the Gizmo on Mystery Powder Analysis and submit it.
Monday, October 23, 2017
Monday/Tuesday, 23/24 October, 2017
ESSENTIAL QUESTION: How has the Atomic Model changed over time?
LEARNING TARGET: Describe how the modern atomic theory was developed.
BENCHMARKS: SC.912.P.8.3
LEARNING OBJECTIVES: Students will be able to:
-Complete the Separation of Mixtures lab write up.
-Describe the atomic theory and the reasons for the changes in the model over time.
-Describe the structure of atoms in terms of protons, neutrons, and electrons, and differentiate among these particles in terms of mass, electrical changes and location within the atom.
BELL RINGER - Choose an element and record the protons, neutrons, and electrons for that element’s atom.
VOCABULARY: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas, notation, nonmetal, octet rule
HOME LEARNING: update notebook
AGENDA
WHOLE GROUP
Students chose an element to share with the class, noting its number of protons, neutrons, and electrons.
We completed the separation of mixtures lab.
Students then did the notes for the atomic theory. Students used the handout below to record their information.
You can find the power point in video form with which to complete the notes below.
Thursday, October 19, 2017
Thursday/Friday, 19/20 October, 2017
ESSENTIAL QUESTION: How has the Atomic Model changed over time?
LEARNING TARGET: Describe how the modern atomic theory was developed.
NGSSS: SC.912.P.8.3
BENCHMARKS:
-Explore the scientific theory of atoms (also known as atomic theory) by describing changes in the atomic model over time and why those changes were necessitated by experimental evidence.
LEARNING OBJECTIVES: Students will be able to:
-Describe the structure of atoms in terms of protons, neutrons, and electrons, and differentiate among these particles in terms of mass, electrical changes and location within the atom.
-Complete the Separation of Mixtures lab write up.
-Research information for project It’s Simply Elemental!.
BELL RINGER - ! and 2 of Atomic structure worksheet
VOCABULARY: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas, notation, nonmetal, octet rule
HOME LEARNING: work on project
AGENDA
WHOLE GROUP
Students completed number one on the handout above, identifying the particles of an atom and their charge.
Students then completed work on their projects.
Subscribe to:
Comments (Atom)