ESSENTIAL QUESTION: How do molecules interact to form covalent bonds?
NGSSS: SC.912.P.8.6; SC.912.P.8.7
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
-Interpret formula representations of molecules and compounds in terms of composition and structure.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and an der Waals forces.
BELL RINGER: Write the name of each of the first 4 compounds. We will complete the page after the videos.
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: study for the mid-year exam.
INFORMATION PRESENTED IN CLASS:
Students completed the remainder of the handout, listing ratio of metal to non-metal elements, the type of bond (single, double, triple), and pasting the compound in the space provided.
Students then made four new compounds and completed the worksheet. Find the worksheets for this section below.
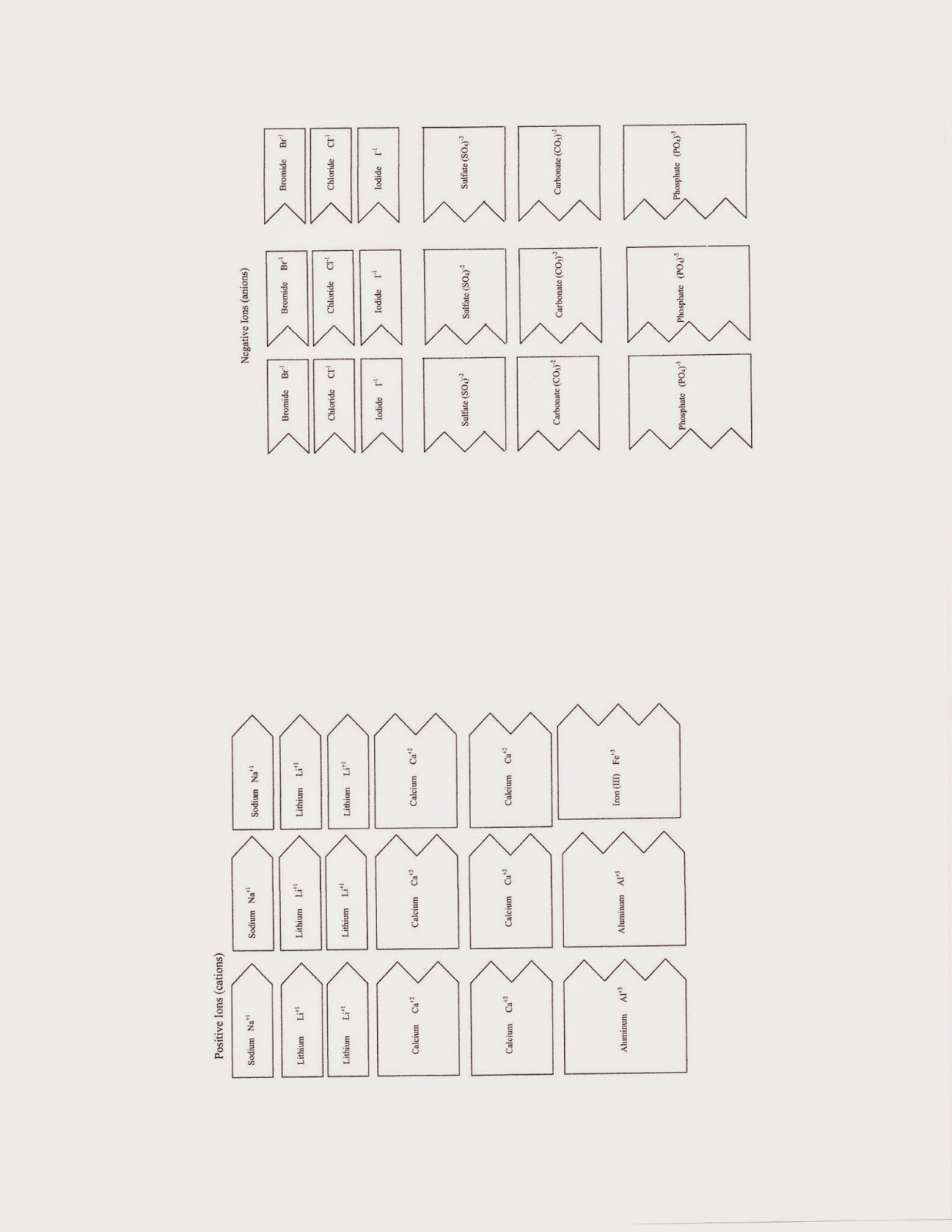 |
| These are the cutouts. I suggest coloring all the cations one color and the anions another color. Don't color so darkly that you can't read what's on the slip. |

