Here is the link for the super taster video.
Super Tasters
Day-to-day activities from Dr. Gayden's Chemistry class at Dorothy M. Wallace COPE Center.
Wednesday, April 22, 2015
Monday, March 2, 2015
Tuesday/Wednesday, 03/04 March, 2015
Click the link below to access the balancing equations activity at Jefferson Labs. Click beginner and 15 equations. Once you've completed the beginner level, continue through intermediate and advanced levels.
Balancing Act!
Once you conquer the Jefferson site, go on to the next site and complete all activities.
Balancing Chemical Equations
Balancing Act!
Once you conquer the Jefferson site, go on to the next site and complete all activities.
Balancing Chemical Equations
Thursday, February 19, 2015
Chemistry Third Grading Period Research Paper/Project
Chemistry Students:
Here are the directions for your third grading period research project/paper. You will chose a chemical from one of the following categories:
Chemicals that make up the human body
Chemicals in foods
Common medications
Chemical reactions in nature
Household cleaners
Safe mosquito repellants
Chemicals of pest control
Hair coloring and perms
You will write a paper about one of the chemicals found in any of the above. Your paper must be printed out in black ink or hand written in blue or black ink on only one side of a page. Be sure to answer the following questions about the chemical you've chose:
1. What is name of the chemical-give its molecular formula and structure.
2. What is the source of this chemical?
3. How was the chemical discovered?
4. What does the chemical do?
5. What class of chemical is it? Is it ionic or covalent? What type of reaction forms the chemical? Is it formed by redox, synthesis, or replacement (single or double)?
6. What are the benefits of this chemical to the world?
7. Are there any deleterious effects of this chemical on people? If so, what are they?
8. Is this chemical available to everyone or to only a select group? Why?
9. How is this chemical disposed of?
Remember, the finished project is due in class on March 17/18.
Remember, the finished project is due in class on March 17/18.
Tuesday, January 20, 2015
Tuesday/Wednesday, 20/21 January, 2015
ESSENTIAL QUESTION: How do molecules interact to form covalent bonds?
NGSSS: SC.912.P.8.6; SC.912.P.8.7
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
-Interpret formula representations of molecules and compounds in terms of composition and structure.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and an der Waals forces.
BELL RINGER: Answer the questions on the handout. You will then enter your answers using the clicker CPS System. I apologize for not including the questions here. My computer is on the fritz and I cannot scan documents on this computer. Once my computer is fixed, I will go back and add any missing pages.
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: study for the mid-year exam.
INFORMATION PRESENTED IN CLASS:
Students reviewed information with the clickers and participated in the discussion. Students then reviewed covalent boding by viewing the Bitesize revision presentation from the UK. Click on the link Covalent Bonding to learn more.
We then took notes on molecular compound formation (formation of compounds with covalent bonds). We will pick up note taking at the next class session.
Wednesday, January 14, 2015
Wednesday/Thursday, 14/15 January, 2015
ESSENTIAL QUESTION: How do molecules interact to form covalent bonds?
NGSSS: SC.912.P.8.6; SC.912.P.8.7
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
-Interpret formula representations of molecules and compounds in terms of composition and structure.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and an der Waals forces.
BELL RINGER: Write the name of each of the first 4 compounds. We will complete the page after the videos.
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: study for the mid-year exam.
INFORMATION PRESENTED IN CLASS:
Students completed the remainder of the handout, listing ratio of metal to non-metal elements, the type of bond (single, double, triple), and pasting the compound in the space provided.
Students then made four new compounds and completed the worksheet. Find the worksheets for this section below.
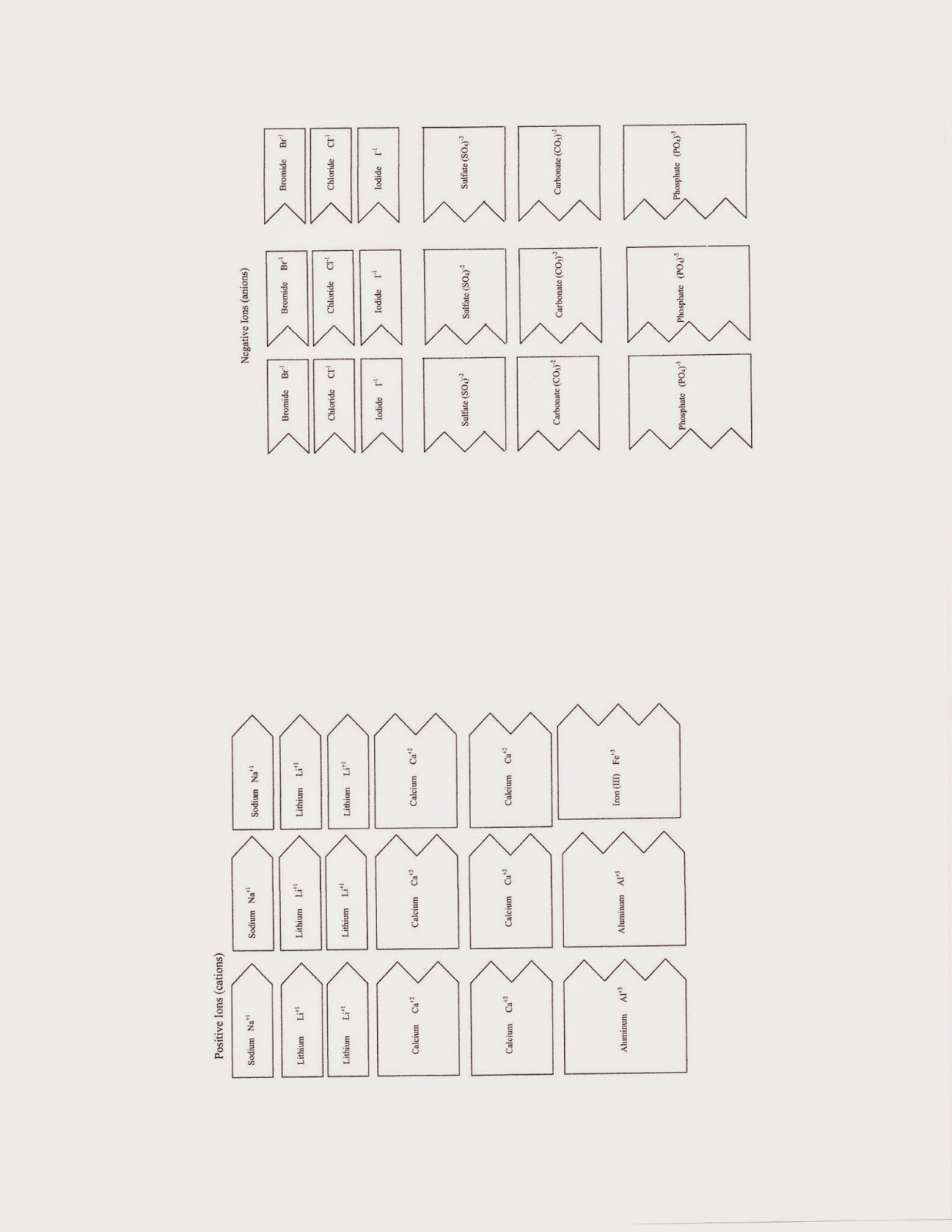 |
| These are the cutouts. I suggest coloring all the cations one color and the anions another color. Don't color so darkly that you can't read what's on the slip. |
Monday, January 12, 2015
Monday/Tuesday, 12/13 January, 2015
ESSENTIAL QUESTION: How do molecules interact to form covalent bonds?
NGSSS: SC.912.P.8.6; SC.912.P.8.7
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
-Interpret formula representations of molecules and compounds in terms of composition and structure.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and an der Waals forces.
BELL RINGER: Read the cartoon and suggest elements for each character. Use your periodic table.
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: HL 7/work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
Students completed the bell ringer and added it to their interactive notebook. Be sure you have placed the date, benchmark, essential question and page number in your table of contents. Then, set up a Cornell notes page with date, page number and essential question. Place the cartoon in the center of the Cornell notes page.
We then reviewed HL 8.
Students then watched the BrainPop movie Chemical Bonds. You can access the movie with the user name: palm beach and password: palm beach.
We then completed a virtual covalent bonding activity, found at Covalent Bonding.
Students then began notes on covalent bonding. The entire powerpoint will be found on My Big Campus. Since we did not complete the entire chapter, I will post the completed power point on My Big Campus and the shortened movie version here when we do complete all four sections.
Make sure all work is submitted by Wednesday.
Thursday, January 8, 2015
Thursday/Friday, 08/09 January, 2015
ESSENTIAL QUESTION: Why do bonds form between elements?
NGSSS: SC.912.P.8.6
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between ionic, covalent bonds, and metallic bonds and explain how they are formed.
BELL RINGER: Complete the five questions from the top of the handout with AT, NT or ST.
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: HL 7/work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
Students completed questions 1-5 on the handout below, discussing why they chose the answers they did.
HL 8. On your OWN paper, answer questions 6-13, placing ONLY the answers that would correctly complete each statement on your paper
Students reviewed home learning 7 and received instructions for HL 8, which can be found above.
We also completed note on ionic and metallic bonding. Remember, if you missed some of the notes, you can find the entire power point on My Big Campus. You can also find the shortened movie version of the notes on this site (see last class blog notes).
We then practiced recognizing bond types. The handout can be found below. Complete and place in your notebook.
Read the directions to write either ionic, covalent, or both for the bond types. You will also need a periodic table! Place the completed handout in your interactive notebook.
Students continued to practice making ionic bonds on their personal devices. Click the link Ionic Bonding to practice this skill.
Students also completed an exit slip on bonding.
Don't forget, projects are due in class on Monday!
Tuesday, January 6, 2015
Tuesday/Wednesday, 06/07 January, 2015
ESSENTIAL QUESTION: Why do bonds form between elements?
NGSSS: SC.912.P.8.6
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between ionic, covalent bonds, and metallic bonds and explain how they are formed.
BELL RINGER: Students were asked to draw the electron dot configurations for water (H2O), salt (NaCl), and carbon dioxide (CO2)
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: HL 7/work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
Students received HL 7, which is to be done on your own paper. Be sure to write your answers on the handout and place in your interactive notebook.
This is the shortened version of the powerpoint, minus much of the explanatory information and examples. To see the entire powerpoint, please go to My Big Campus.
We will conclude notes next class.
Don't forget, papers and projects are due next Monday!
Home learning is due next class!
Friday, December 19, 2014
Friday, 19 December, 2014 and Monday, January 5, 2015
ESSENTIAL QUESTION: Why do bonds form between elements?
NGSSS: SC.912.P.8.6
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between ionic, covalent bonds, and metallic bonds and explain how they are formed.
BELL RINGER: Students were given handout and asked to draw the Lewis dot configuration of the valence electrons.
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
After reviewing the bell ringer, we reviewed home learning 6. B sure to place both the bell ringer and the home learning in your interactive notebook.
Students then viewed the video Dogs Teaching Chemistry on forming bonds. They did a stop and jot as the video played. You can find the video below.
 |
| Use this sheet and the information from the video to help you complete the sheet below. |
Wednesday, December 17, 2014
Wednesday/Thursday, 17/18 December, 2014
ESSENTIAL QUESTION: Why do bonds form between elements?
NGSSS: SC.912.P.8.6
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between ionic, covalent bonds, and metallic bonds and explain how they are formed.
BELL RINGER: Use your phone to look up chemical formula for the vitamin you wrote about during the lat class. Also find the structural formula for your vitamin. Record both in your notes
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: HL 6: Ionic and Metallic Bonding/work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
Students completed the bell ringer by writing the chemical formula and the structural formula for their chosen vitamin in their interactive notebook.
We then discussed how the the structural formula is how the molecule looks, while the chemical formula contains all the parts of the molecule. We noted that none of the vitamin molecules would be possible if the various elements could not bind together to make the compounds.
Students received HL 6, which can be found below:
Monday, December 15, 2014
Monday/Tuesday, 15/16 December, 2014
ESSENTIAL QUESTION: Why do bonds form between elements?
NGSSS: SC.912.P.8.6
BENCHMARK(S):
-Distinguish between bonding forces holding compounds together and other attractive forces, including hydrogen bonding and van der Waals forces.
LEARNING OBJECTIVES: Students will be able to:
-distinguish between ionic, covalent bonds, and metallic bonds and explain how they are formed.
BELL RINGER: Use your phone to look up a disease caused by a vitamin deficiency. You will prepare a poster depicting the vitamin and explaining how the lack of or low levels of that vitamin affect the body. You will also include a printed picture on your poster.
VOCABULARY: valence electron electron dot structure, octet rule, halide ion, ionic bond, ionic compound, chemical formula, formula unit, coordination number, metallic bond, alloy
HOME LEARNING: work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
Students completed a poster about a disease caused by a vitamin deficiency. The poster should include a short description of the disease and how the lack of the particular vitamin causes the disease. It should also include a picture of someone affected with the disease.
Due to many students completing the exam, this is as far as we got in the lesson. For those who like to forge ahead, read chapter 7 and begin writing (and illustrating) al the vocabulary listed above.
Due to many students completing the exam, this is as far as we got in the lesson. For those who like to forge ahead, read chapter 7 and begin writing (and illustrating) al the vocabulary listed above.
Thursday, December 11, 2014
Thursday/Friday, 11/12 December, 2014
ESSENTIAL QUESTION: Exam
NGSSS: SC.912.P.10.9; SC.912.P.10.18
BENCHMARK(S):
-Describe the quantization of energy at the atomic level.
-Explore the theory of electromagnetism by comparing and contrasting the different parts of the electromagnetic spectrum in terms of wavelength, frequency, and energy, and relate them to phenomena and applications.
LEARNING OBJECTIVES: Students will be able to:
-take an exam on the quantum mechanical theory and electromagnetic spectrum.
BELL RINGER: NA
VOCABULARY: NA
HOME LEARNING: work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
Thursday was an early release day, therefore classes were shortened. There was also a writing boot camp given during the morning. Because of these factors, students were given the entire class time to work on their exam. Friday classes should expect to spend a comparable amount of time (about an hour) before moving onto other activities.
For the even block classes on Friday, students will do differentiated instruction, either individually using Edgenuity or work with the instructor to review information on which they received the lowest scores. Students should bring tablets to class!
Tuesday, December 9, 2014
Tuesday/Wednesday, 09/10 December, 2014
ESSENTIAL QUESTION: Exam
NGSSS: SC.912.P.10.9; SC.912.P.10.18
BENCHMARK(S):
-Describe the quantization of energy at the atomic level.
-Explore the theory of electromagnetism by comparing and contrasting the
different parts of the electromagnetic spectrum in terms of wavelength,
frequency, and energy, and relate them to phenomena and applications.
LEARNING OBJECTIVES: Students will be able to:
-take an exam on the quantum mechanical theory and electromagnetic spectrum.
BELL RINGER: Students were given a 15 minute study session to ask last minute questions and review class content.
VOCABULARY: NA
HOME LEARNING: work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
INFORMATION PRESENTED IN CLASS:
Students took a chapter exam on the quantum mechanical theory and electromagnetic spectrum.
Students who are absent or out for medical/delivery will take the exam when they return to school.
Students who are absent or out for medical/delivery will take the exam when they return to school.
Friday, December 5, 2014
Friday/Monday, 05/08 December, , 2014
ESSENTIAL QUESTION: What is the electromagnetic spectrum?
NGSSS: SC.912.P.10.18
BENCHMARK(S):
-Explore the theory of electromagnetism by comparing and contrasting the different parts of the electromagnetic spectrum in terms of wavelength, frequency, and energy, and relate them to phenomena and applications.
LEARNING OBJECTIVES: Students will be able to:
-explore the theory of electromagnetism by comparing and contrasting the different parts of the electromagnetic spectrum.
-describe the electromagnetic spectrum in terms of frequency, wavelength and energy.
BELL RINGER: Students answered questions about the EM Spectrum as their bellringer. Questions 1-10 were reviewed. The site with the questions is:Electromagnetic Spectrum Quiz
VOCABULARY: wavelength, amplitude, frequency, hertz, crest, trough
HOME LEARNING: Study for the exam on Chapters 5 and 6 (periodic table, history of atomic theory, quantum theory, electromagnetic spectrum);/work on your science fair paper/project
INFORMATION PRESENTED IN CLASS:
INFORMATION PRESENTED IN CLASS:
-We reviewed the bell ringer and home learning 5.
-Students completed the Cornell notes, with their summary and teacher-like questions. Use the added pages in the back of your notebook to help you write quality questions. Be sure your summary is inclusive of all you learned about the topic, not just a basic statement.
-Data chats were concluded.
-Students attempted to work on a virtual lab on the electromagnetic spectrum, but technological differences made that impossible. Those that wish to complete the activity at home will receive extra credit. Visit Dr. Gayden's Science Zone, and click on the link for chemistry students to access the lab. You can find the handouts below.
-Don't forget to study for the exam! See home learning above to know what information from your text and notes to review.
 |
| Add caption |
 |
These are the handouts to accompany the online activity. Complete for extra credit. |
Wednesday, December 3, 2014
Wednesday/Thursday, 03/04 December, 2014
ESSENTIAL QUESTION: What is the electromagnetic spectrum?
NGSSS: SC.912.P.10.18
BENCHMARK(S):
-Explore the theory of electromagnetism by comparing and contrasting the different parts of the electromagnetic spectrum in terms of wavelength, frequency, and energy, and relate them to phenomena and applications.
LEARNING OBJECTIVES: Students will be able to:
-explore the theory of electromagnetism by comparing and contrasting the different parts of the electromagnetic spectrum.
-describe the electromagnetic spectrum in terms of frequency, wavelength and energy.
BELL RINGER: Complete the Wavestown handout by listing all the examples in the town that represent each of the wave types of the electromagnetic spectrum. The handout tells you how many examples of each there are. There are multiple uses for the observatory.
VOCABULARY: wavelength, amplitude, frequency, hertz, crest, trough
HOME LEARNING: work on your science fair paper/project AND HL 5 Wave Speed
INFORMATION PRESENTED IN CLASS:
After completing the Wavestown handout, we practiced solving wavelength problems, which is the home learning assignment, as listed below. Be sure to write the answers to all the questions on your handout to be placed in your notebook. Write the answers, and show your work on a separate sheet of paper to submit for a grade. The home learning sheet is listed below.
Students then participated in the Pass It On Activity. Students received a sticky with one of the vocabulary words listed at the top. Students wrote one fact on the sticky and passed it on to another students so that student could write a fact. Each student should have written comments on a minimum of three stickies.
We added pages to our interactive notebook A contact information sheet was placed in the back inside cover. Pages a through e were labeled in the back of the notebook. A new table of contents page was added to show that these new pages are indeed.
Handouts explaining in detain how to write the Cornell notes questions were distributed and should be used to help students increase achievement. These were placed on pages 5 and 6 of the notebook.
Data chats were began, and students worked individually (Khan's Academy videos-see the list you were given) or CK-12.org assignments as their classmates were called up for the data chap.
INFORMATION PRESENTED IN CLASS:
After completing the Wavestown handout, we practiced solving wavelength problems, which is the home learning assignment, as listed below. Be sure to write the answers to all the questions on your handout to be placed in your notebook. Write the answers, and show your work on a separate sheet of paper to submit for a grade. The home learning sheet is listed below.
 |
| This is HL 5. Answers on your own paper. Be sure to show your work and include the correct unit! |
Students then participated in the Pass It On Activity. Students received a sticky with one of the vocabulary words listed at the top. Students wrote one fact on the sticky and passed it on to another students so that student could write a fact. Each student should have written comments on a minimum of three stickies.
We added pages to our interactive notebook A contact information sheet was placed in the back inside cover. Pages a through e were labeled in the back of the notebook. A new table of contents page was added to show that these new pages are indeed.
Handouts explaining in detain how to write the Cornell notes questions were distributed and should be used to help students increase achievement. These were placed on pages 5 and 6 of the notebook.
Data chats were began, and students worked individually (Khan's Academy videos-see the list you were given) or CK-12.org assignments as their classmates were called up for the data chap.
Subscribe to:
Posts (Atom)








