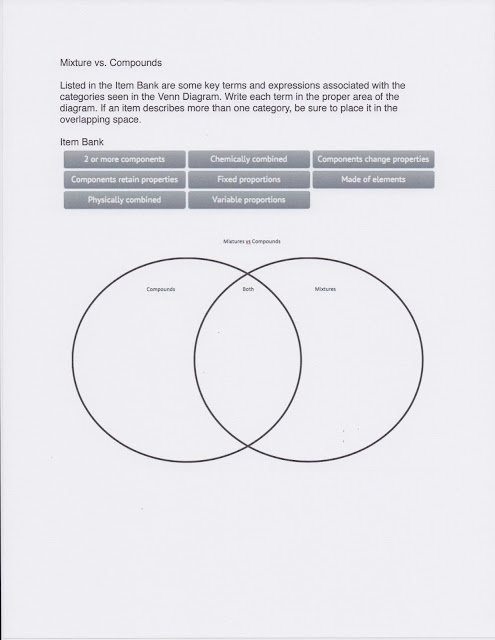
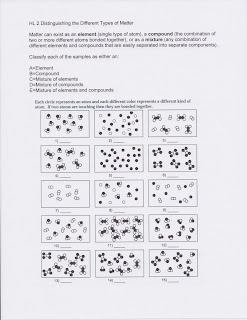
Benchmark: SC.912.N.1.1
Essential Question: How can we use the scientific method to solve problems?
Learning Objectives: By the end of this lesson, I should be able to:
-Evaluate a scientific investigation using evidence of scientific thinking and problem solving.
-Differentiate between replication and repetition and evaluate the use and need for each in a scientific investigation.
Bell Ringer: None
VOCABULARY: observations, systematic observations, experiment, test (independent variable), outcome (dependent variable), data, empirical evidence, predict, hypothesis, trials, repetition, replication, control group, conclusion
Home Learning: notebook update
AGENDA
WHOLE GROUP
-update notebook, including Costa's leveled questions, color, and summary.
-Question 1-What are the steps of the scientific method?
-Question 2-Compare and contrast independent and dependent variables.
-Question 3-Design an experiment that uses the scientific method to solve a problem.
Be sure to color coordinate the questions and answers. Use a different highlighter for each question. See me if you have any questions.
Also, place a summary on the bottom of page 11. It should read:
The scientific method is a way to solve problems. The steps include asking a question, doing research, forming a hypothesis, doing an experiment, making observations and gathering data, analyzing results, drawing conclusions and sharing findings.
SMALL GROUP/INDEPENDENT PRACTICE/DI
-drops on a penny lab. Use the scientific method to investigate how the difference of the sides of the penny affects the number of drops that can fit.
You should have:
Drops on a Penny
Problem: Which side of a penny holds the most drops of water?
Hypothesis: If drops of water are placed on a penny, then the (heads/tail) side will hold the most drops because.....)
Materials: cup, water, dropper, penny, paper towel
Procedures:
1) Fill a dropper with water.
2) Place a penny heads up on a table.
3) Add water to the surface of the penny. Count each drop as it is added. Be sure NOT to touch the tip of the dropper to the growing dome of water on the penny. STOP adding when the water spills off the penny.
4) Repeat for the tails side of the penny.
Variables;
Independent Variable: Side of the penny
Dependent Variable: Number of drops added
Constants: same penny, same dropper, same liquid
Data and Observations
Results: The _________ side of the penny held more water.
Conclusions:
Claim: The _________ side of the penny held the most drops of water.
Evidence: The heads side held ______ drops of water. The tails side held _____ drops of water. This was a difference of _______ drops.
Reasoning: Surface tension is the ability of water molecules to stick to each other. Since the penny is made of the same material on both sides, the surface tension should be equal on both sides. The difference in the number of drops added, if any, cold be due to errors in experimentation.
Exit Strategy - Orally review one thing you learned today in class.