ESSENTIAL QUESTION: How has the Atomic Model changed over time?
LEARNING TARGET: Describe how the modern atomic theory was developed.
BENCHMARKS: SC.912.P.8.3
LEARNING OBJECTIVES: Students will be able to:
-Describe the structure of atoms in terms of protons, neutrons, and electrons, and differentiate among these particles in terms of mass, electrical changes and location within the atom.
-Student data chat.
BELL RINGER - Complete the handout Your guide to the atom
VOCABULARY: alkali metals, alkaline earth metals, group (family), halogen, inner transition (rare earth metals), ion, metal, metalloid, noble gas, notation, nonmetal, octet rule
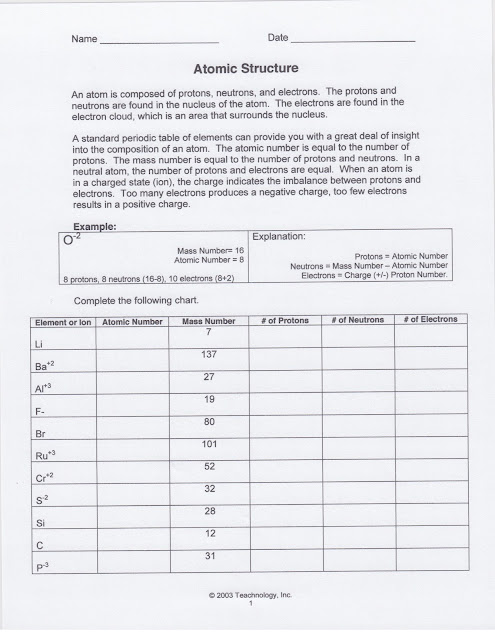
HOME LEARNING: HL 1: Atomic Structure
AGENDA
WHOLE GROUP
Students completed the handout Your Guide to the Atom.
Students received HL 1 Atomic Structure. It is due next class period. You can find the copy of the handout above.
We reviewed how to determine the number of protons, neutrons, and electrons in both charged and neutral atoms. We also determined how to calculate the number of neutrons using the atomic mass of the element.
Students then worked on the Bohr's Introduction model Gizmo. Be sure to complete the five question assessment at the end fo the GIZMO.